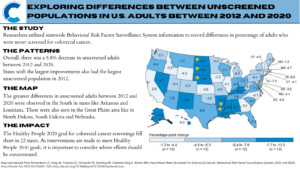
Cancer screening remains a powerful tool. Even limited screening has long-term benefits compared to no screening and can lower the risk of cancer and related deaths. A recent study by researchers at the CDC compared data on adults who reported they had not received a colorectal cancer (CRC) screening test between 2012 and 2020 using information from the Behavioral Risk Factor Surveillance System (BRFSS). The study identified various trends, most notably that 22 states did not meet the CDC’s Healthy People 2020 goal of 70.5% adults screened for CRC.
The sample was limited to adults aged 50 to 75 years, with up to date screenings defined as one of the following:
- Home stool-blood test within the past year
- Sigmoidoscopy within five years with fecal occult blood test or within one year with fecal immunochemical test
- Colonoscopy within ten years
The ‘never screened’ numbers were a composite of those who answered no to being screened or those who were not up to date. Those who declined to answer or reported uncertainty were excluded. Overall, the study identified:
- A 5.8% decrease in unscreened adults between 2012 and 2020
- States with the largest improvements were also those with the largest unscreened population in 2012
Despite these improvements, CRC screening goals have yet to be met and may be difficult to meet with the new Healthy People 2030 standards. The target of 74.4% screened may have been a challenge to meet, possibly further exacerbated by the COVID-19 pandemic.
Researchers noted that including just two more questions on the BRFSS in 2020, the percentage of up to date screenings increased to 71.6%. These two questions enquired about:
- Stool DNA testing
- Computerized tomographic colonography
It is important to note that the National Colorectal Cancer Roundtable—a membership organization established by the CDC and the American Cancer Society—has set its goal to 80% screening rates across the country.
Study authors recognized recall bias and an inability to distinguish between screening versus diagnostic tests as major study limitations. Additionally, social desirability bias and a low response rate may have also affected the results. However, financial factors and health disparities may also describe the differences between states.
Following implementation of the Affordable Care Act, researchers at the American Cancer Society found that CRC screening among low-income adults across the U.S. increased by up to 8%, with the greatest increases observed in early Medicaid expansion states. They also noted that a majority of those who were never screened also lived in a state without expansion (South Dakota).
Nonfinancial factors such as health disparities were studied in a mixed-methods analysis conducted at the Virginia Commonwealth University’s School of Medicine. Here, researchers noted that participants of gender-specific and race-specific focus groups brought forth nuanced concerns regarding screening. This included lack of awareness of both the disease and the screening, lack of physician recommendation that is clear and rational, and fear of being diagnosed and complications associated with testing. These concerns, if unaddressed, may limit others from seeking out CRC screening.
To read more about the Healthy People 2030 CRC screening standards and the current progress, visit Healthy People 2030.
Kaylinn Escobar is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.

Early-Age Onset Colorectal Cancer: Health Disparities in the Black Population
Colorectal cancer (CRC) is a leading cause of cancer-related deaths in the U.S., and the incidence of early-age onset CRC (EAO-CRC)—when the disease is diagnosed in those younger than 50 years—is rising. In the Black population, EAO-CRC makes up nearly 10% of all new diagnoses. The incidence of EAO-CRC in the Black population (8.4 cases per 100,000 people in 2019) is slightly lower than that of the White population (8.9 cases per 100,000 people in 2019), but this is reversed when it comes to the mortality rate. The mortality rate of EAO-CRC in the Black population is 2.4 deaths per 100,000 people, while it is 1.8 deaths per 100,000 people in the White population. Between 2015-2019, 5,329 new EAO-CRC cases were diagnosed among Black Americans.
Studies have shown that Black individuals are more likely to be diagnosed with EAO-CRC at a younger age and a more advanced stage than White individuals: 22% of White Americans receive a metastatic diagnosis compared with 26% of Black Americans.
While additional research is needed to discern the higher incidence of EAO-CRC in the Black population, certain socioeconomic and environmental factors likely play an important role. These include limited access to proper healthcare services, the prevalence of food deserts leading to poor nutrition, and living in areas with high pollution rates. Additional resources to support research, prevention, and treatment efforts of EAO-CRC in this population are critical.
Empowering the population via awareness and education campaigns around the early warning signs of CRC and the importance of screening in the Black community would also go a long way. Early warning signs of CRC include changes in bowel movements, blood in stool, unexpected weight loss, and continuous abdominal discomfort. If you are experiencing these symptoms, speak with your doctor.
Additional information on prevention, symptoms, and diagnosis of CRC can be found under ‘Resources’ on the Colon Cancer Foundation’s website.
Emma Edwards is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Can Circulating Tumor DNA Predict Who Will Benefit from Colorectal Cancer Chemotherapy?
Over 30% of patients with stage II or stage III colorectal cancer (CRC) and 60-70% of patients who undergo oligometastatic resection experience cancer recurrence. Stage II or III CRC is usually treated with surgery followed by adjuvant chemotherapy (ACT). However, patients with clinical and pathological risk factors only see a 10-15% decrease in cancer recurrence with standard ACT.
Now, a new study proposes using circulating tumor DNA (ctDNA) as a predictive biomarker to guide chemotherapy treatment decisions in CRC patients.
ctDNA is a minimally invasive biomarker that can help oncologists measure disease status and progression during cancer therapy, including the detection of molecular residual disease (MRD). In this study, researchers evaluated whether ctDNA following surgery could predict disease recurrence in early-stage CRC.
The study enrolled 1,563 patients with:
Blood samples were collected before and at predetermined time intervals after surgery (up to 18 months), and imaging was performed every six months until 18 months after surgery. MRD, defined as ctDNA positivity after surgery or therapy, is strongly associated with poor prognosis in patients with surgically resectable CRC. Of the 1,039 patients included in the ctDNA analysis, 18.0% were ctDNA positive four weeks after surgery.
Researchers discovered that patients with high-risk stage II, stage III, and stage IV CRC, who were ctDNA-positive four weeks after surgery, benefited from ACT. ctDNA was identified as the most significant risk factor for CRC recurrence in these patients, and ctDNA positivity is an important predictor of ACT benefit.
Regardless of the pathological stage of CRC, patients with a higher risk of recurrence based on ctDNA status may benefit from ACT, while those with negative ctDNA status may be able to avoid unnecessary ACT. These findings can guide clinicians in making evidence-based treatment decisions for CRC patients.
Sahar Alam is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
CDC Report: Why Do Certain Populations Never Screen for Colorectal Cancer?
Cancer screening remains a powerful tool. Even limited screening has long-term benefits compared to no screening and can lower the risk of cancer and related deaths. A recent study by researchers at the CDC compared data on adults who reported they had not received a colorectal cancer (CRC) screening test between 2012 and 2020 using information from the Behavioral Risk Factor Surveillance System (BRFSS). The study identified various trends, most notably that 22 states did not meet the CDC’s Healthy People 2020 goal of 70.5% adults screened for CRC.
The sample was limited to adults aged 50 to 75 years, with up to date screenings defined as one of the following:
The ‘never screened’ numbers were a composite of those who answered no to being screened or those who were not up to date. Those who declined to answer or reported uncertainty were excluded. Overall, the study identified:
Despite these improvements, CRC screening goals have yet to be met and may be difficult to meet with the new Healthy People 2030 standards. The target of 74.4% screened may have been a challenge to meet, possibly further exacerbated by the COVID-19 pandemic.
Researchers noted that including just two more questions on the BRFSS in 2020, the percentage of up to date screenings increased to 71.6%. These two questions enquired about:
It is important to note that the National Colorectal Cancer Roundtable—a membership organization established by the CDC and the American Cancer Society—has set its goal to 80% screening rates across the country.
Study authors recognized recall bias and an inability to distinguish between screening versus diagnostic tests as major study limitations. Additionally, social desirability bias and a low response rate may have also affected the results. However, financial factors and health disparities may also describe the differences between states.
Following implementation of the Affordable Care Act, researchers at the American Cancer Society found that CRC screening among low-income adults across the U.S. increased by up to 8%, with the greatest increases observed in early Medicaid expansion states. They also noted that a majority of those who were never screened also lived in a state without expansion (South Dakota).
Nonfinancial factors such as health disparities were studied in a mixed-methods analysis conducted at the Virginia Commonwealth University’s School of Medicine. Here, researchers noted that participants of gender-specific and race-specific focus groups brought forth nuanced concerns regarding screening. This included lack of awareness of both the disease and the screening, lack of physician recommendation that is clear and rational, and fear of being diagnosed and complications associated with testing. These concerns, if unaddressed, may limit others from seeking out CRC screening.
To read more about the Healthy People 2030 CRC screening standards and the current progress, visit Healthy People 2030.
Kaylinn Escobar is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Neoadjuvant Immunotherapy Effective for Mismatch Repair–Deficient Colorectal Cancer
Mismatch repair–deficient (dMMR) or microsatellite instability–high (MSI-H) colorectal cancer (CRC) is an advanced form of CRC that is highly responsive to treatment with immunotherapy, especially PD-1 inhibitors. Preliminary research results demonstrate that PD-1 inhibitors are significantly effective cancer treatments, with high response rates and sustained progression-free survival.
A new study investigated the treatment impact of neoadjuvant PD-1 inhibitors on the long-term survival of dMMR CRC patients. The study found that PD-1 inhibitor treatment before surgery was significantly effective among patients with dMMR/MSI-H CRC.
Seventy-three patients with dMMR/MSI-H CRC who had previously been treated with PD-1 inhibitors were included in a retrospective review. The most common locations of primary tumors were in the rectum (24.7%) and ascending colon (24.7%). 79.5% of patients were treated with PD-1 inhibitor alone. The study found:
These findings are promising for patients with nonmetastatic dMMR/MSI-H CRC, including those with locally advanced disease. Dustin A. Deming, MD, University of Wisconsin Carbone Cancer Center, stated in an NCCN newsletter, “The treatment of mismatch repair deficient locally-advanced colorectal cancer is a highly active area of research. This retrospective analysis highlights the potential for significant treatment responses with limited toxicities for these patients treated with immune checkpoint inhibitors. It will be exciting to see how these results, and other completed and ongoing studies, will be utilized to incorporate anti-PD1 treatments into the standard-of-care for locally-advanced colorectal cancers.”
To read more about types of immunotherapy drugs and their impact on cancer care, visit Understanding Cancer Immunotherapy Research.
Sahar Alam is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Plant-Based Diets Lower Colorectal Cancer Risk in Men
Colorectal cancer (CRC), the third most common cancer and the third leading cause of cancer-related deaths in the U.S., is preventable with regular screening. In addition to routine screening, other modifiable risk factors, such as diet, play an important role in lowering the risk of CRC. For example, red and processed meats are associated with an increased risk for CRC, while diets rich in dietary fiber reduce the risk of CRC.
A recent prospective cohort study discovered that plant-based diets rich in healthy plant foods were associated with a lower risk of CRC in men, and varied based on race, ethnicity, and tumor location. These findings signify the importance of incorporating healthy plant foods into diets and reducing meat consumption to lower the risk of CRC.
The participants completed a food frequency questionnaire with over 180 food items. PDI, hPDI, and uPDI were calculated based on scoring methods and defined food groups that included:
Each food group was associated with specific scores.
The study found that a plant-based diet that includes natural, rather than processed, plant-based foods is associated with a reduced risk of CRC in men. For women, however, none of the plant-based diets were significantly associated with CRC risk. For both men and women, the average scores of PDI and hPDI were highest among Japanese Americans and lowest among Native Hawaiians. The mean uPDI was highest in Native Hawaiian men and lowest in African American men and white women. Men with higher scores for PDI and hPDI had a 24% and 21% lower risk of CRC than men with lower scores for those diets, respectively. Furthermore, no significant association was found between risk for CRC and uPDI for men.
These analyses highlight the potential significance of plant-based diets in preventing CRC and suggest that the benefits of plant-based diets can vary based on sex and race/ethnicity. The findings underscore the importance of increasing healthy and less-processed plant foods in our diet and reducing meat consumption to lower the risk of CRC.
Sahar Alam is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Masculinity May be a Barrier to Completing Colorectal Cancer Screening
Colorectal cancer (CRC) mortality is preventable with regular screening. Differences in early detection screening rates lead to disparities in CRC mortality among White, Black, and American Indian/Alaska Native (AIAN) men. Complicating the issue of racial disparity observed with CRC screening rates is the psychosocial aspect of men’s health. A recent study investigated the impact of masculinity barriers on CRC screening and found that they influence CRC screening completion.
This survey-based cross-sectional study analyzed the association between the male thought process and the successful completion of CRC screening tests. Male respondents aged 18 to 75 years from across the U.S. who self-identified as Black, AIAN, or white were surveyed. Four Masculinity Barriers to Medical Care subscale were investigated:
The highest score, which translates into the greatest barrier for screening, was for “Being strong” and “Negative attitudes towards medical professionals and exams”
Lower scores were observed for “Acknowledging emotions and health issues” and “Positive attitudes toward medical professionals and exams”
For all men, “being strong” was associated with a 54% decreased odds of CRC screening completion.
Reluctancy to seek and engage in preventive health services, such as CRC screening, due to fear of presenting as weak or vulnerable is associated with men who strongly support masculine ideals. The investigation also demonstrated that Black men who scored higher on negative attitudes toward medical professionals and exams subscale had lower odds of CRC screening uptake. The sensitivity analysis of the study reflected that AIAN men had lower odds of CRC completion than Black men.
Medical mistrust is positively correlated with masculine ideologies. Despite decreasing CRC incidence and mortality rates in adults ages 50 years and older, early-onset CRC has increased among adults younger than 50 and is predicted to increase by 90% by 2030. Considering the above masculinity barriers in future population-based and intervention research is critical for increasing men’s participation in CRC screening.
Sahar Alam is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Have Colonoscopy Rates Among 45-49 Year Olds Improved After Guideline Change?
Initiating regular screening at the recommended age and time interval is key to preventing and early diagnosis of colorectal cancer (CRC). An early CRC diagnosis improves survival. The American Cancer Society (2018) and the U.S. Preventive Services Task Force (2020) recommended that CRC screening should start at 45 instead of 50 years, in reaction to the growing incidence of CRC among younger people.
All major U.S. guidelines now endorse average-risk CRC screening at 45 years of age. However, there are concerns that endoscopic capacity may be strained, that low-risk persons may self-select for screening, and that calculations of the adenoma detection rate may be diluted.
A new study supports the recommendation that colonoscopies should start at age 45, not 50 years. In this study, researchers compared colonoscopy volumes and lesion detection rates in the U.S. healthcare system before (October 2017 to December 2018) and after (January 2019 to August 2021) the new guidelines were issued. They included 7,990 patients who had undergone colonoscopies from October 2017 through August 2021: 4,266 first-time colonoscopies and 3,724 re-screening colonoscopies.
Researchers found that:
They concluded that in our healthcare system in the early contemporary era of updated CRC screening guidelines, screening colonoscopy volume among 45- to 49-year-old patients has increased modestly, and lesion detection rates in 45- to 49-year-old patients have not decreased as might have been seen if low-risk persons were self-selecting for screening. The authors acknowledged that their findings were based on a single healthcare system and that national data will be important to assess the impact of the revised guidelines.
Kitty Chiu is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Expanding Multigene Panel Testing Criteria for Colorectal Cancer Patients
Multigene panel testing (MGPT) is a tool to identify genetic mutations that can increase an individual’s risk of a disease such as cancer. MGPT can also be used to develop a treatment plan or to help predict whether cancer will spread to other parts of the body. A recent study examined colorectal cancer (CRC) patients and found that 14.2% of patients carried at least one pathogenic germline variant (PGV), with more than one PGV identified in 1.4% of patients.
Identification of pathogenic or likely PGVs in hereditary cancer predisposition genes can affect a patient’s treatment plan. While there is increased support for universal MGPT for certain forms of cancer, eligibility criteria for CRC are more restrictive: germline genetic testing for CRC is recommended only for a subset of patients with CRC who meet certain “high-risk criteria,” which include:
The above mentioned study conducted MGPT across a diverse CRC population to determine whether genetic testing criteria for patients with CRC should be broadened. The results of the study found that of the 34,244 individuals who were tested:
This research study is the largest to date examining MGPT in CRC, and demonstrated high rates of clinically actionable variants detected, independent of the age at the time of testing, the number of genes on the panel, and race/ethnicity. These findings provide evidence to support the broadening of genetic testing criteria for patients with CRC, which in turn will have a significant impact on disease management, the treatment plan, and ultimately, disease outcome.
What Do I Need to Know About Genetic Testing for Colorectal Cancer?
Genetic factors play an important role in the development of colorectal cancer (CRC). Some people have inherited genetic syndromes that increase their risk for colon cancer. Genetic testing looks for these inherited syndromes along with changes in DNA that are associated with a greater likelihood of developing cancer.
What is Genetic Testing for CRC?
Genetic testing looks for changes in your DNA that are known to be associated with an increased risk of cancer. Generally, there are two ways that genetic testing may be used:
According to the American Cancer Society, genetic tests can help show if members of certain families have inherited a high risk of CRC due to inherited cancer syndromes such as Lynch syndrome (also known as hereditary non-polyposis colorectal cancer, or HNPCC) or familial adenomatous polyposis (FAP).
Who is Considered “High-Risk”?
Those with a family history of CRC may benefit from speaking to their primary care physician, oncologist, or surgeon about the importance of genetic testing to identify if there was a mutated gene that predisposes them to cancer. You may be a good candidate for genetic testing for CRC if you have:
What Can I Expect With the Procedure?
If your doctor believes that you’re a good candidate for genetic testing, they’ll likely refer you to a genetic counselor. Genetic testing is typically done using a blood sample. However, it may also use a sample of saliva, cheek cells, or skin. This sample will be sent to a specialized lab that will run the test. After a few weeks, your results will be sent over to your doctor or genetic counselor and you’ll be contacted to discuss your test results and next steps.
How Much Does Genetic Testing for Colon Cancer Cost and is it Covered by Insurance?
Genetic testing can be expensive and can cost between $100 to over $2,000, depending on the nature and complexity of the test. Many insurance providers will cover the cost of genetic testing and genetic counseling if it’s considered medically necessary.
Nevertheless, always check with your insurance provider to see what’s covered before getting tested. For additional information about insurance coverage, please visit: Paying for Genetic Services.
Kitty Chiu is a Colorectal Cancer Prevention Intern with the Colon Cancer Foundation.
Clinical Decisions Support Systems May Exacerbate Healthcare Disparities
Clinical decision support systems (CDSS) are computer-based applications used to analyze data within electronic health records (EHRs). CDS algorithms are progressively being integrated into healthcare systems to expand patient care. However, research and development in ethical frameworks have uncovered that CDS applications can perpetuate bias in healthcare. A recent EHR quality improvement study has revealed significant differences in family history accessibility, availability, and comprehensiveness based on sex, race and ethnicity, and language preference. These findings propose that historically medically underserved populations are excluded from identification from CDS tools based on family history information, unintentionally reinforcing existing healthcare disparities and potentially creating more disparities in healthcare systems.