The Colon Cancer Foundation (CCF) spoke with Dr. Rami James Aoun, 11th winner of the Dr. Thomas K. Weber Colorectal Cancer Research Scholar Award, for his work looking at biomarkers of radiation response in rectal cancer patients. He is a surgical resident at The Ohio State Wexner Medical Center. Instituted in 2011 by CCF and the Society of Surgical Oncology to recognize translational research focused on the molecular biology of colorectal cancer, the award was renamed in 2020 to honor CCF’s founder, the late Dr. Thomas K. Weber.
Born in West Palm Beach, Florida, Dr. Aoun was raised in Beirut, Lebanon, where he was a student at the American University of Beirut. After completing his undergraduate years and medical school, Dr. Aoun joined Columbia University in New York where he received a Master of Public Health degree in Healthcare Management and Policy. As part of his ongoing residency at The Ohio State Wexner Medical Center, he is completing a research fellowship with Dr. Matthew Kalady, a colorectal surgeon at The James Cancer Center.

Dr. Rami James Aoun
Q: What motivated you to work in the oncology research space, and colorectal cancer in particular?
Dr. Aoun: I am motivated to work in oncology research because I have seen some of my own family members suffer from cancer. However, what specifically interests me in colon cancer research are the patients that I encounter here at The James Cancer Center and my mentors. Their guidance when I was a junior resident was extremely important to set the direction for me as a future colorectal surgeon. That’s how I met Dr. Kalady, and now I am a part of his lab conducting research on colorectal cancer, with the goal of improving patient care outcomes.
Q: Can you summarize the significance of your findings for which you have received this award? Can you also share the prior work or observations that laid the foundation for this project?
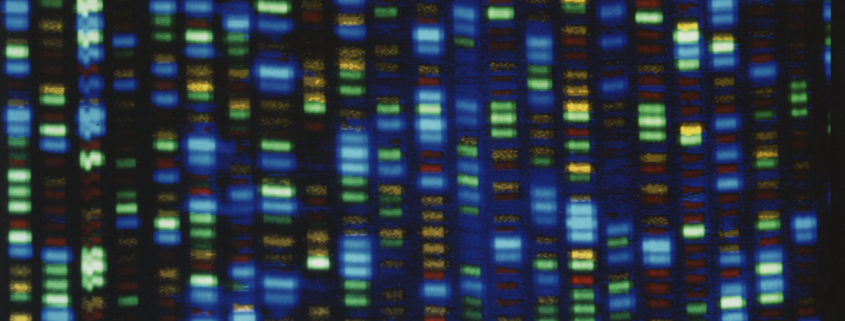
Dr. Aoun: We observed a difference in how patients with rectal cancer reacted to neoadjuvant radiation therapy. Some of the patients who were exposed to neoadjuvant therapy had a complete response—the cancer disappeared. However, there were patients who had almost no response to the therapy. The response can be determined and graded by examining the tumor under a microscope. Patients who had a better response end up living longer without cancer.
We sought to identify the reason certain cancers responded to neoadjuvant radiation and certain cancers did not. To do that, we tried to understand these cancers at the genetic level by studying how a rectal cancer expressed particular genes, as measured by mRNA. By comparing the gene expression in both, patients who responded to radiation therapy and those who did not, we were able to obtain a gene signature that helps us identify patterns of gene expression that are different between responders and non-responders.
While this is just a starting point, it can help us develop a more predictive model to use clinically. Once we validate this model, we could be able to distinguish between a responder and non-responder to radiation based on the gene expression that we obtained from their biopsies even before any treatment is administered. This would allow us to provide individualized patient-specific therapy and avoid any unnecessary treatments and procedures.
We also think that certain genes in this signature can be further studied to see if they might be able to be blocked or changed to improve the response to treatment.
Q: What was the size of your current cohort and what is the ‘n’ that you are looking for to be able to validate your study results?
Dr. Aoun: Our ‘n’, or sample size, was 33 patients for this study. In genetic studies like this, it is difficult to design a statistical power needed to validate, but we hope to test this in about 100 different patients.
Q: Did you see any commonality in the gene signatures between rectal cancer and colon cancer?
Dr. Aoun: The gene signature we investigated was related to radiation resistance in rectal cancer, whereas colon cancer is not usually treated with radiation therapy. So, we did not study this for colon cancer. However, some of the pathways we identified are known to be relevant to colon cancer. In terms of the common pathways, what we know is the WNT pathway specifically is involved in the development and progression of colon cancer and rectal cancer. In the gene signature that we identified, six of the genes are involved in the WNT pathway. So, the question is whether the WNT pathway is also involved in radiation resistance in rectal cancer.
Q: Rectal cancer has been steadily increasing in the younger population. Do we know why that may be happening?
Dr. Aoun: An increasing number of younger patients are being afflicted with colorectal cancer and we don’t fully know why. There are lots of different theories about diet, lifestyle, and the microbiome (i.e. the bacterial content in the colon and rectum). This is a hot area of research and many groups are trying to figure out this question.