It is finally here! The U.S. Preventive Services Task Force (USPSTF)’s final recommendation has lowered the colorectal cancer (CRC) screening age for average-risk adults from 50 to 45 years. This long-awaited final recommendation came a little over six months after the draft recommendation was released in October last year.
This is a B grade recommendation, meaning that there is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. Screening for 50–75-year-old adults remains an A grade recommendation, meaning USPSTF believes there is high certainty of substantial net benefit with screening that age group. The recommendations have also been published in JAMA.
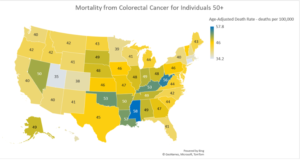
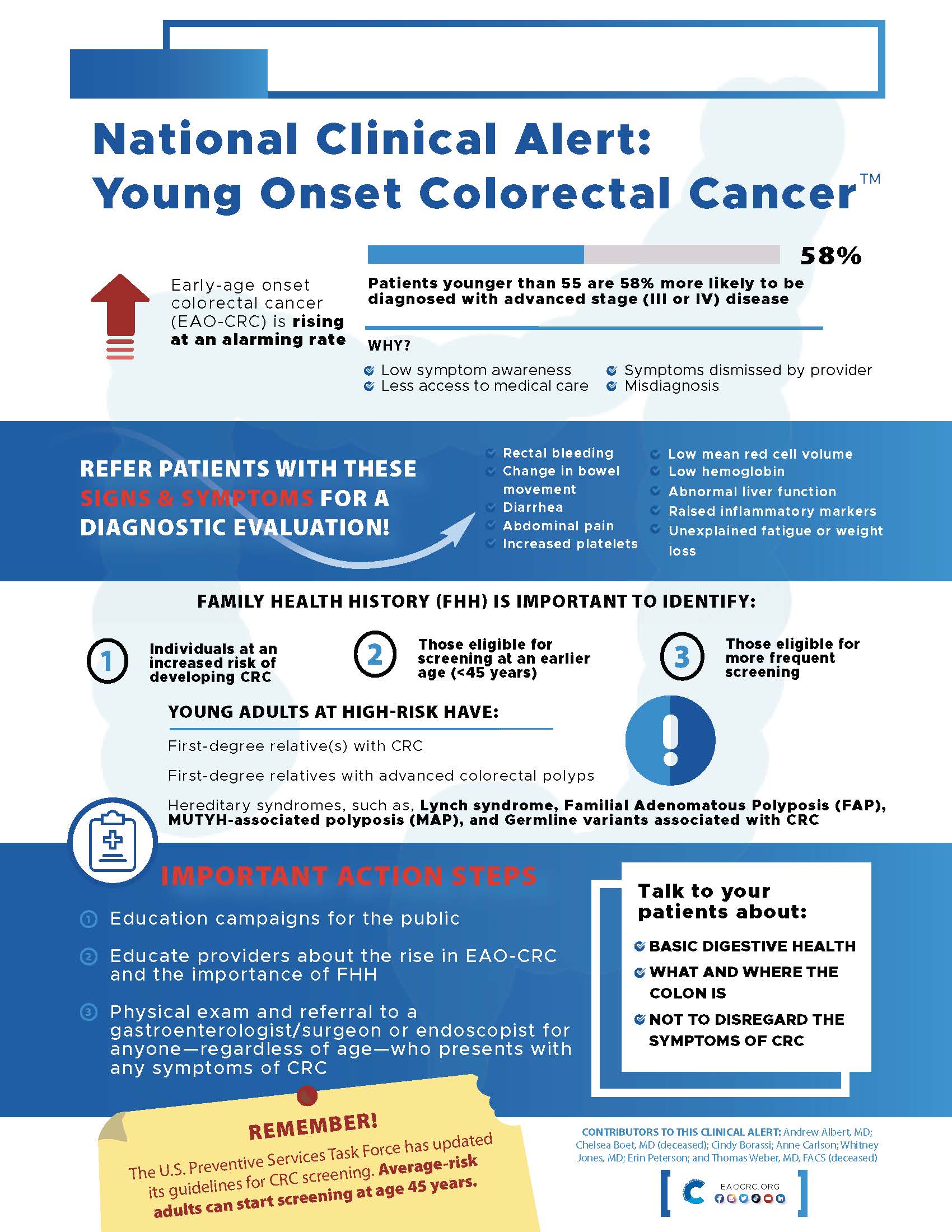
Lowering the screening age from 50 to 45 years is great news for the CRC community because it will significantly influence the earlier diagnosis of CRC among the younger age group. To bring this into perspective, a recent paper in JAMA Network Openprojected that by 2040, CRC will be the second most common cancer in the 20-49 age group and it will be the leading cause of cancer-related deaths in that age group.
Ana Acuna-Villaorduna, MD, Department of Medical Oncology at Montefiore Health System, believes that the lowering of screening age could halt the alarming rise in early-age onset CRC, particularly among racial and ethnic minority populations. “Considering US-based population projections that foresee a continuous increase in racial/ethnic minorities and have higher frequencies and worse clinical courses of colorectal cancer among young patients, it is necessary to adopt a screening strategy aimed to halt this alarming trend,” Dr. Acuna-Villaorduna wrote in an email.
She believes that general practitioners and family physicians will be strategic players in implementing the new measures by educating patients in the community, along with gastroenterologists and medical oncologists. However, uptake of these recommendations by the primary care clinical community may be slow, depending on the messaging strategies that are utilized.
“As usual, it will take a while to get the message out,” Zuri A. Murrell, MD, FASCRS, Chair, Cancer Committee, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, told the Colon Cancer Foundation. “Having a media blitz will be helpful. Organizations like the American Cancer Society and the Colon Cancer Foundation will be the main people who will not only get the word out to providers, but to the community as well,” he added.
Dr. Acuna-Villaorduna thinks that the USPSTF recommendation will finally build a consensus across the various medical societies that have disparate CRC screening recommendations. She believes that some physicians may already have been screening average-risk adults at 45 years, so guideline uptake may be faster.
What Else Will Influence Uptake of the Guidance?
Another important question is whether our health care system has the capacity to onboard the 20-21 million adults in the 45-49 age group who are now eligible to get screened. While colonoscopy remains the gold standard, other screening options, including stool-based testing, could be used to conduct the initial screen. Whitney Jones, MD, Founder, Colon Cancer Prevention project, agrees. “While we cannot conduct colonoscopy in all the new population, we can definitely send them stool-based testing kits. That’s what health systems should focus on,” he said while speaking with the Colon Cancer Foundation.
The other issue is insurance coverage for CRC screening as a preventive care service for the 45-49 age group and making sure payers—both government and private—are aware of the updated guidelines and are integrating these within their policy.
Currently, most insurers will cover the cost of a preventive screening test for those 50 years or older, but the enrollee may have to share the cost of a diagnostic screening test if a polyp or tumor is found.
If historical trends are any indication, insurance coverage of colonoscopy will significantly influence increased screening in the younger age group. Evidence for this is stark in the Medicare population: colonoscopy rates jumped from 20% in 2000 to 61% in 2018 among those 50 and older after Medicare started covering colonoscopy screening for all beneficiaries in 2001.
Once the updated recommendations of CRC screening age are implemented, Dr. Murrell is hopeful that insurance companies will listen and hopefully start covering the cost of the simple preventive procedure. He also raised an important point about eliminating fear from the mind of the younger community. “I have coined a slogan that I always share with my patients: ‘You shouldn’t die from fear you shouldn’t die from embarrassment.’ This will be the first step in helping and encouraging people to get a colonoscopy,” he added.